Abstract
Background: Childhood cancer has an ~80% cure rate but comes at the cost of long-term secondary health consequences. Clonal hematopoiesis (CH) is a consequence of aging and a precursor to myeloid malignancies. The presence of CH in adults confers an increase in all-cause mortality, predominately thrombotic events, including stroke and myocardial infarction. The Childhood Cancer Survivor Study (CCCS) has demonstrated the phenomenon of accelerated aging in childhood cancer survivors-24-year-old survivors of childhood cancer have the same cumulative incidence of grade 3-5 health conditions as a 50-year-old sibling. Currently there is no earlier method of detection or risk stratification for these patients other than screening complete blood counts (CBC) at yearly clinic visits. To date, clonal hematopoiesis has not been prospectively studied in this population in an unbiased fashion and represents a critical knowledge gap which may help us better care for this population.
Rational and Hypothesis: To determine the rate of clonal hematopoiesis in childhood cancer survivors. We hypothesize that the accelerated aging experienced by pediatric survivors promotes clonal hematopoiesis and confers an increased risk of leukemia/myelodysplasia.
Methods: Consented and enrolled 300 eligible patients from our survivorship clinic. Processed peripheral blood samples using a Ficoll-Paque Premium to isolate peripheral blood mononuclear cells (PBMCs) within 72 hours of collection. gDNA was isolated using Qiagen DNeasy and CH-associated mutations were identified using a hybridization capture-based targeted sequencing panel covering 24 genes predicted to encompass >95% of CH mutations observed in adults (Vanderbilt VANTAGE). Sequencing coverage of >500x allowed for variant frequency detection down to 1%. Raw data was aligned with BWA-MEM and analyzed with the Terra Bio CHIP Detection workspace following GATK best practice guidelines.
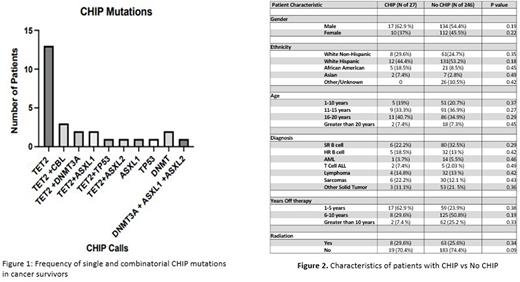
Results: 273 patients had a CHIP analysis completed We were able to enroll a representative cohort of pediatric cancer survivors. The age of our patient sample ranged from 3 to 30 years and time from therapy ranged from 2 to 20 years. Patients had a wide variety of chemotherapy exposures as well as diagnoses. Of the 273 patient samples that were analyzed we had 63 mutations in 27 unique patients. These mutations included Nonsynonymous SNV, Stopgain and Frameshift mutations in TET 2, DNMT3A, ASXL1 and ASXL2, CBL and TP53. This was a rate of 9.9% which is much higher than what is reported in the literature for general population studies in adults.
Conclusions: Childhood cancer and its treatment is associated with an increased risk for clonal hematopoiesis in genes such as TET2 and DNMT3A, genes implicated in cardiovascular events and drivers of myeloid malignancy. These events, while rare, suggest a vulnerability that with further exploration/characterization will aide in the prevention/treatment of these devastating late effects.
Disclosures
Kroger:Takeda: Consultancy, Honoraria; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding; DKMS: Research Funding; Riemser: Research Funding; Neovii: Honoraria, Research Funding; Amgen: Honoraria; Kite: Honoraria; Jazz: Honoraria; Sanofi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal